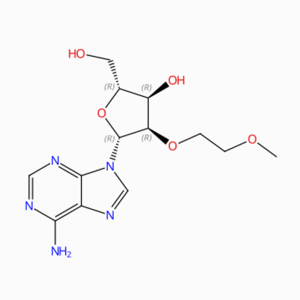
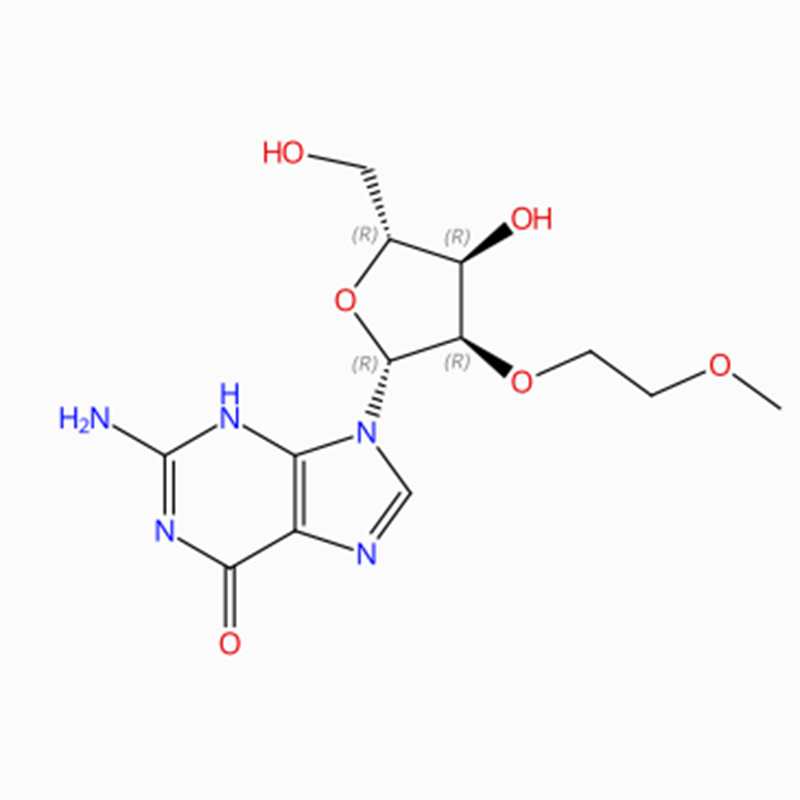
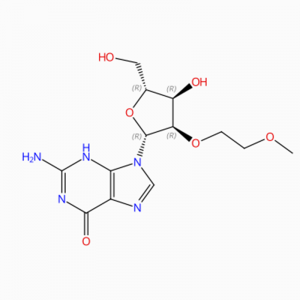
C13H19N5O6 Guanosinum, 2′-O-(2-methoxyethylum)- (9Cl, ACI)
Numerus Registri CAS
473278-54-5
| Proprietates Physicae Claves | Valor | Conditio |
| Pondus Moleculare | 341.32 | - |
| Punctum Ebullitionis (Praedictum) | 715.0±70.0°C | Prelum: 760 Torr |
| Densitas (Praedicta) | 1.81±0.1 g/cm³ | Temperatura: 20°C; Pressio: 760 Torr |
| pKa (Praedictum) | 13.20±0.70 | Temperatura Acida Maxima: 25°C |
Subrisus Canonici
O = C1N = C(N)NC2 = C1N = CN2C3OC(CO)C(O)C3OCCOC
Subrisus Isomerici
O(CCOC)[C@H]1[C@@H](O[C@H](CO)[C@H]1O)N2C3=C(N=C2)C(=O)N=C(N)N3
InChI
InChI= 1S/C13H19N5O6/c1-22-2-3-23-9-8(20)6(4-19)24-12(9)18-5-15-7-10(18)16-13(14)17-11(7)21/h5-6,8-9,12,19-20H,2-4H2,1H3,(H3,
Clavis InChI
DLLBJSLIKOKFHE-WOUKDFQISA-N
1 Aliud Nomen pro hac Substantia
2′ -O-(2-Methoxyethyl)guanosinum (ACI)
Proprietates praesto
Optica et Dispersio
Thermalis
| Possessio | Valor | Conditio | Fons |
| Potentia Rotatoria Optica | -51 deg-mL/g-dm² | c: 1.4 g/100ml; Solvens: Dichloromethanum; λ: 589.3 nm; Temperatura: 25°C | (1) CAS |
| Potentia Rotatoria Optica | Vide Textum Integrum | (2) CAS |
(1) Wen, Ke; Acta Chemiae Organicae, (2002), 67(22), 7887-7889, CAplus.
(2) Wen, Ke; Acta Chemiae Organicae, (2002), 67(22), 7887-7889, CAplus.
| Possessio | Valor | Fons Conditionis |
| Punctum Liquefactionis | Vide Textum Integrum | (1) CAS |
(1) Taj, Shabbir Ali S.; Nucleosida, Nucleotida et Acida Nucleica, (2008), 27(9), 1024-1033, CAplus.
Spectra praesto sunt
1H NMR
13C NMR
IR
Proprietates praesto
Biologicus
Chemica
Densitas
Lipinski
Structurae Relata
Thermalis
| Possessio | Valor | Conditio | Fons |
| Factor Bioconcentrationis | 1.0 | pH 1; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 2; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 3; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 4; Temperatura: 25°C | (1) ACD |
| Possessio | Valor | Conditio | Fons |
| Factor Bioconcentrationis | 1.0 | pH 5; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 6; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 7; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 8; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 9; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 1.0 | pH 10; Temperatura: 25°C | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Conditio | Fons |
| Koc | 1.0 | pH 1; Temperatura: 25°C | (1) ACD |
| Koc | 1.0 | pH 2; Temperatura: 25°C | (1) ACD |
| Koc | 4.27 | pH 3; Temperatura: 25°C | (1) ACD |
| Koc | 8.49 | pH 4; Temperatura: 25°C | (1) ACD |
| Koc | 9.43 | pH 5; Temperatura: 25°C | (1) ACD |
| Koc | 9.54 | pH 6; Temperatura: 25°C | (1) ACD |
| Koc | 9.55 | pH 7; Temperatura: 25°C | (1) ACD |
| Koc | 9.55 | pH 8; Temperatura: 25°C | (1) ACD |
| Koc | 9.55 | pH 9; Temperatura: 25°C | (1) ACD |
| Koc | 9.54 | pH 10; Temperatura: 25°C | (1) ACD |
| logD | -2.65 | pH 1; Temperatura: 25°C | (1) ACD |
| logD | -1.84 | pH 2; Temperatura: 25°C | (1) ACD |
| logD | -1.08 | pH 3; Temperatura: 25°C | (1) ACD |
| logD | -0.78 | pH 4; Temperatura: 25°C | (1) ACD |
| logD | -0.74 | pH 5; Temperatura: 25°C | (1) ACD |
| logD | -0.73 | pH 6; Temperatura: 25°C | (1) ACD |
| logD | -0.73 | pH 7; Temperatura: 25°C | (1) ACD |
| logD | -0.73 | pH 8; Temperatura: 25°C | (1) ACD |
| logD | -0.73 | pH 9; Temperatura: 25°C | (1) ACD |
| logD | -0.73 | pH 10; Temperatura: 25°C | (1) ACD |
| logP | -0.730±0.568 | Temperatura: 25°C | (1) ACD |
| Solubilitas Intrinseca Massae | 0.44 g/L | Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 38 g/L | pH 1; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 5.5 g/L | pH 2; Temperatura: 25°C | (1) ACD |
| Possessio | Valor | Conditio | Fons |
| Solubilitas Massae | 0.96 g/L | pH 3; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.48 g/L | pH 4; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | pH 5; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | pH 6; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | pH 7; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | pH 8; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | pH 9; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | pH 10; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.44 g/L | Aqua non tamponata pH 7.20; Temperatura: 25°C | (1) ACD |
| Solubilitas Intrinseca Molaris | 1.3 × 10⁻³ mol/L | Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 0.11 mol/L | pH 1; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 0.016 mol/L | pH 2; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 2.8 × 10⁻³ mol/L | pH 3; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.4 × 10⁻³ mol/L | pH 4; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | pH 5; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | pH 6; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | pH 7; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | pH 8; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | pH 9; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | pH 10; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.3 × 10⁻³ mol/L | Aqua non tamponata pH 7.20; Temperatura: 25°C | (1) ACD |
| Pondus Moleculare | 341.32 | ||
| pKa | 13.20±0.70 | Temperatura Acida Maxima: 25°C | (1) ACD |
| pKa | 3.00±0.20 | Temperatura Maxima Fundamentalis: 25°C | (1) ACD |
| Pressio Vaporis | 1.86 × 10-21 Torr | Temperatura: 25°C | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Conditio | Fons |
| Densitas | 1.81±0.1 g/cm³ | Temperatura: 20°C; Pressio: 760 Torr | (1) ACD |
| Volumen Molare | 188.5±7.0 cm³/mol | Temperatura: 20°C; Pressio: 760 Torr | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Conditio | Fons |
| Obligationes libere rotabiles | 8 | (1) ACD | |
| Acceptores H | 11 | (1) ACD | |
| Donatores H | 5 | (1) ACD | |
| Summa Donatoris/Acceptoris H | 16 | (1) ACD | |
| logP | -0.730±0.568 | Temperatura: 25°C | (1) ACD |
| Pondus Moleculare | 341.32 |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Fons Conditionis |
| Area Superficialis Polaris | 153 A2 | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Conditio | Fons |
| Punctum Ebullitionis | 715.0±70.0°C | Prelum: 760 Torr | (1) ACD |
| Enthalpia Vaporizationis | 109.72±3.0 kJ/mol | Prelum: 760 Torr | (1) ACD |
| Punctum Inflammationis | 386.2±35.7°C | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
Spectra praesto sunt
1H NMR
13C NMR


![C17H19N3O6 Thymidinum, α-oxo- α-[(phenylmethyl)amino]- (ACI)](https://www.csnvchem.com/uploads/C17H19N3O6-Thymidine-300x300.png)
![C10H12N2O5 6H-Furo[2′,3′:4,5]oxazolo[3,2-a]pyrimidin-6-onum, 2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxymethyl)-7-methyl-, (2R,3R,3aS,9aR)- (9Cl, ACI)](https://www.csnvchem.com/uploads/C10H12N2O5-6H-Furo-300x300.jpg)