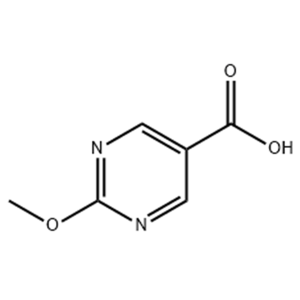
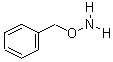
O-Benzylhydroxylamine Hydrochloride 95%
Appearance: O-benzylhydroxylamine hydrochloride is a white to off-white crystalline solid.
Solubility: Soluble in water, soluble in ethanol, the solution is acidic
Stability: O-benzylhydroxylamine hydrochloride is relatively stable at room temperature, but it is susceptible to heat and light, and is easily decomposed. It is not acid-resistant.
Melting point (ºC): undetermined
Flash point (ºC): undetermined
It is a compound with various chemical properties. Some of its main chemical properties are as follows:
Nucleophilic substitution reaction: O-benzylhydroxylamine hydrochloride has nucleophilic substitution reactivity and can be substituted by electron-deficient compounds such as acylating agents, aromatic amides, and aldehydes to generate various different compounds.
Reduction reaction: O-benzylhydroxylamine hydrochloride can be reduced to the corresponding amine by reducing agents, such as sodium bisulfite and hydrogen, to yield benzamidine.
Acylation reaction: O-benzylhydroxylamine hydrochloride can be used to generate important organic intermediates such as acyl hydrazides and imidazolyl hydrazides through acylation reactions.
Acid-catalyzed reaction: O-benzylhydroxylamine hydrochloride can undergo various reactions under acidic conditions, such as condensation reaction, dehydration reaction, and cyclization reaction.
Metal ion-catalyzed reaction: O-benzylhydroxylamine hydrochloride can form complex reactions with metal salts to generate organometallic compounds with special functions.
Photochemical reaction: O-benzylhydroxylamine hydrochloride can undergo photochemical reactions, such as photolysis reaction under UV light, to generate compounds such as nitrosobenzamide.
Storage Condition
Store in an airtight container at room temperature.
Package
Packed in 25kg /drum, lined with double plastic bag, or packed according to customer needs.
Application Fields
It is an important organic synthetic intermediate, which is commonly used for the preparation of hydrazides, imidazoles, and other nitrogen-containing heterocyclic compounds, as well as certain drugs and pesticides.
In addition to being an important intermediate in chemical synthesis, O-Benzylhydroxylamine hydrochloride also has other applications. For instance, it can be used as a processing aid for rubber, which can increase the rate and extent of rubber vulcanization. Furthermore, it can be used as a surfactant, which can enhance the interfacial activity and stability of liquids.
O-Benzylhydroxylamine hydrochloride is a very important organic synthetic intermediate, widely used in the fields of pharmaceuticals, pesticides, dyes, fragrances, rubber, and surfactants.




![methyl 2,2-difluorobenzo[d][1,3]dioxole-5-carboxylate](https://www.csnvchem.com/uploads/carboxylate1-300x300.png)
![Pyrrolo[2,3-d]pyrimidin-4-ol 98%min](https://www.csnvchem.com/uploads/Pyrrolo23-dpyrimidin-4-ol1-300x300.jpg)